In a previous article, we considered traction motors of EVs. In this lecture, we will look into one of the very important elements of an electric car namely, the source of energy, to be more precise, the high-voltage traction battery.
We will consider the main parameters of the traction battery, such as:
- Energy density.
- Battery life.
- Battery efficiency.
- Battery capacity.
Of course, we will also consider the different types of batteries that are used in electric vehicles.
So for now, electric vehicle batteries are one of the most expensive elements of an EV. It can be up to half the cost of the entire electric vehicle. Also, the distance that an electric vehicle can travel on a single charge depends directly on the battery capacity. Of course, the range of one charge is influenced by many factors. but the capacity of the battery is decisive.
Traction battery provides power for the electric vehicle operation the battery gives a constant voltage to the power electronics unit. The power electronics unit converts the DC voltage to AC and feeds the motor-generator.
Main parameters of the traction battery
Energy Density
The first parameter is Energy Density. This parameter allows us to conclude the power of the battery. Energy density is the amount of energy that can be stored in a given mass of a substance. The unit of energy density is watt-hours per kilogram (W⋅h/kg). The range of an electric vehicle can be determined from the energy density.
Battery Life
Another important issue is battery life. The battery life is described by the parameter of resistance to multiple discharge-charge cycles. depending on the conditions of use including the ambient temperature to understand the reduction of battery life, we should consider a specific electric vehicle.
Currently, car manufacturers offer a warranty on their batteries. For example, concerning the Nissan Leaf, the manufacturer offers a warranty on the battery and the electric motor for up to five years or 60,000 miles. Renault’s warranty covers the Zoe electric hatchback for a distance of up to 100,000 miles or three years. Tesla offers an eight-year warranty on the S model which is independent of mileage and can be transferred between the owners.
Incidentally, the battery life is reduced if the motorist constantly uses fast charging technology. Charging the battery with the help of devices that restore up to 80% charge in 30-60 minutes can speed up the process of degradation of the power source by 1.5 to 2 times for the battery to last longer. it is recommended to leave it connected to the charger for several hours.
For example, at night.
Thus, it is possible to reduce the charging current, and, consequently, heating of the elements of the traction battery.
Battery Efficiency
The next parameter is the efficiency of the traction battery. The efficiency of the rechargeable traction battery is indicated as a percentage. Simply put, efficiency shows how much energy spent on charging can be reused when the battery is discharged. Because a small part of the charging energy is dissipated in the form of thermal energy. it means loss during charging the efficiency of the battery can never be equal to 100%. Virtually, every electric car company uses a unique type of battery.
Battery Capacity
Accumulators differ in capacity and provide a different drive range. And although the maximum distance that an electric car can travel also depends on its design, weight, type of electric motor, and the like this figure can be used to compare the batteries.
| Car Model | Battery Capacity in kWh | Cruising Range in km |
|---|---|---|
| Audi e-Tron | 95 | 400 |
| BMW i3 | 33 | 200 |
| Chevrolet Bolt EV | 60 | 300 |
| Chevrolet Spark EV | 19 | 132 |
| Detroit Electric | 37 | 280 |
| Hyundai Ioniq EV | 28 | 200 |
| Hyundai Kona | 64 | 480 |
| JAC iEV7S | 39 | 300 |
| Kia Soul | 64 | 391 |
| Jaguar I-Pace | 90 | 480 |
| Nissan e-NV200 Combi | 40 | 170 |
| Nissan Leaf | 62 | 385 |
| Renault Kangoo ZE | 33 | 270 |
| Renault Zoe | 41 | 367 |
| Smart ForTwo Electric Drive | 17.6 | 160 |
| Tesla Model 3 | 75 | 320 |
| Tesla Model S | 100 | 600 |
| Tesla Model X | 100 | 475 |
| Volkswagen e-Golf | 24.2 | 170 |
| Volkswagen e-Up | 18.7 | 160 |
Types of rechargeable batteries
Now let’s move to the types of rechargeable batteries. Different types of rechargeable batteries differ in the materials used to make electrodes and electrolytes. In a nutshell, we will say what the battery consists of.
Liquid cell batteries consist of three main components:
- The Anode.
- The Cathode.
- The Electrolyte.
Both the anode and cathode are the types of electrodes. They are the conductors through which electricity enters or leaves the component in the circuit.
An electrolyte is a substance, often a liquid or gel capable of transporting ions between chemical reactions that occur at the anode and the cathode.
There are also solid-state batteries. That means that in this type of battery, not a liquid, but a solid is used as an electrolyte. So the type of battery depends on the materials that are used as the cathode, the anode, and the electrolyte.
Liquid cell batteries
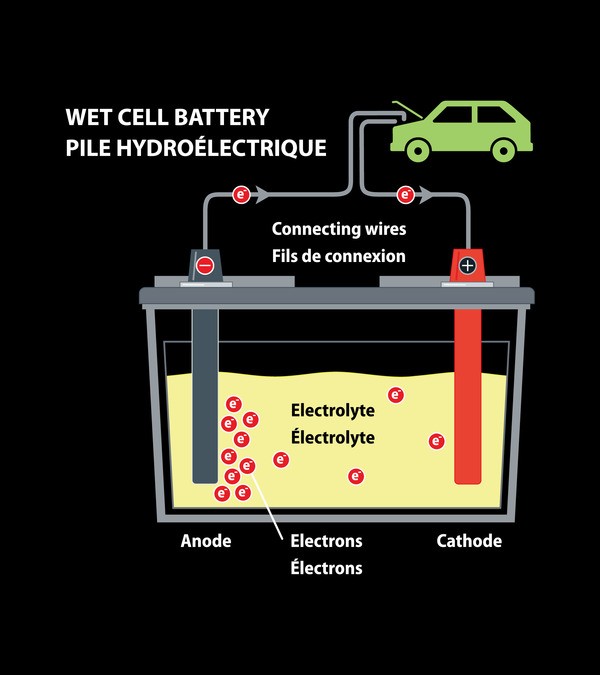
Lead batteries
A lead battery is a classic 12-volts rechargeable battery to power the onboard network of the car. Lead and lead oxide plates serve as electrodes, and sulfuric acid solution serves as the electrolyte.
Lead batteries require maintenance. This means that they need to add distilled water to ensure the necessary level of electrolyte. Lead-acid batteries are not suitable for powering vehicles moving, exclusively on electrical traction. The reason for this is their very large mass. Such a battery would take up most of the car’s volume and this would reduce the working load of the car.
After 6 years, a lead battery can lose most of its electrical capacity. Also, in case of damage, an electrolyte acid may leak from it.
Dry cell batteries
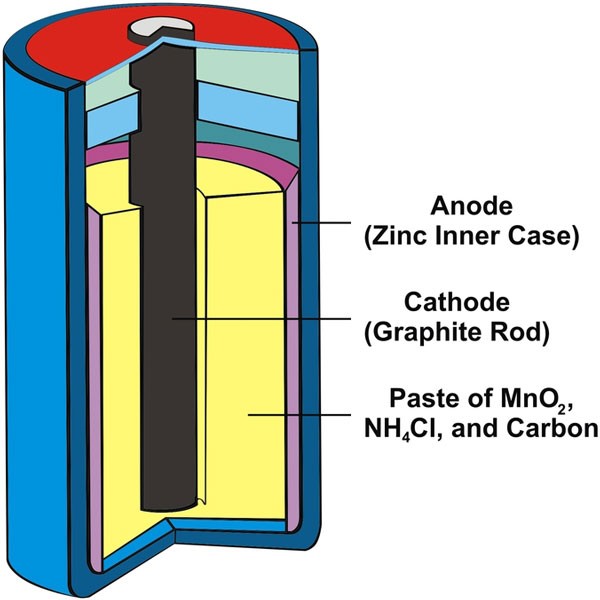
Nickle-cadmium batteries
The next type of traction battery is a nickel-cadmium battery. These batteries use cadmium and a nickel compound as an electrode material. the electrolyte is potassium hydroxide.
Therefore, this type of battery is also called alkaline batteries. They have a higher energy density than lead batteries and are more resistant to damage and electrolyte leakage.
But they have two huge drawbacks that make them practically unsuitable for full use in electric cars.
- Cadmium and cadmium compounds are poisonous.
- Nickel-cadmium batteries have a memory effect.
What is the memory effect in batteries?
The memory effect, also known as the “lazy battery” effect is an effect that causes the battery to accept and hold less charge. That is, batteries gradually lose their maximum energy capacity if they repeatedly recharge after incomplete discharge. Thus, the battery remembers the smaller capacity of the battery than it was first.
Nickel-metal hydride batteries
Next comes the Nickel-Metal Hydride battery. in these batteries, a nickel compound and a different metal compound are used as the material for the electrodes. Potassium hydroxide is also used as an electrolyte.
They have a higher energy density than nickel-cadmium batteries and are relatively resistant to damage. The memory effect of nickel-cadmium batteries is less pronounced. But even with these batteries, over time the efficiency is reduced these efficiency losses are reversible to a certain degree.
The advantage of nickel-metal hydride batteries is that they do not contain toxic heavy metals like lead or cadmium. The electrolyte in the battery is contained in a solid form. Even if the housing is destroyed, only separate sprays are possible.
Lithium-ion batteries
Finally, we turn to lithium-ion batteries. these batteries are the most modern and common in electric vehicles. Various lithium metal and graphite oxides are used as electrodes and various solvents for lithium salts are used as the electrolyte.
Lithium-ion (Li-ion) batteries contain only very small amounts of water and do not have a memory effect. Compared with nickel-cadmium batteries, they have almost twice the energy density. This means that this type of battery requires less space for installation in an electric vehicle. so there is more free space for passengers and the luggage compartment.
But, with strong heating in the battery, the process of disintegration may begin. This can cause fire and the release of gases that are harmful to health. Therefore, when handling these batteries, it is necessary to strictly follow the manufacturer’s safety instructions.
Incidentally, the word “lithium” is derived from the Greek “lithos” which means stone, because it was discovered in 1817 in stone.
A lithium-ion battery is not a specific type of battery with a single approved composition, but a whole family of batteries with different compositions of electrochemical cells. Each type of lithium-ion battery is good for a particular application. And of course, not all of them are used in electric transport. Let’s look at those that are used as a traction battery.
Lithium-manganese batteries
The first type that we will look into is the Lithium-manganese battery. Lithium-manganese battery, due to the three-dimensional structure, can provide a high discharge current which is up to 30 times greater than its capacity. This type of battery is used in electric cars Nissan Leaf, Chevy Volt, and BMW i3.
But lithium-manganese batteries have some drawbacks such as a small life span, and intolerance to cold. Therefore, lithium-manganese batteries are combined with another type of battery. Lithium-nickel-manganese-cobalt-oxide battery (NMC). So, let’s talk about them.
Lithium-nickel-manganese-cobalt-oxide batteries (NMC)
NMCs have good specific energy consumption and service life of up to 2000 charge/discharge cycles. but their recoil current is low. That is why to be used in electric vehicles.
NMC is combined with the lithium-manganese battery during normal driving, NMC cells mainly operate, and during acceleration, Lithium-manganese battery cells give off a high current.
Lithium-nickel-cobalt-aluminum oxide batteries (NCA)
NCA batteries have a high specific capacity and an acceptable cost. Charge rate and discharge current for NCA batteries are average. It was the NCA that became the energy source for Tesla cars and Powerwall storage systems. Incidentally, it was the use of these batteries that cast a shadow on Tesla’s company.
After all, this battery has a service life of 500 cycles. The real experience has shown that even after 200 thousand kilometers of battery, Tesla electric vehicles remain working, losing a third of their capacity.
Lithium titanate batteries (LTO)
LTO battery is under development by Toshiba. They produce this type of battery which is called “SCiB“, which means Super Charge Ion Battery.
In 2017, Toshiba demonstrated a SCiB-battery capable of recovering up to 90% of its capacity in just 5 minutes. Lithium-titanate batteries stably deliver a current ten times their capacity, and thirty times with pulsed loads Modern samples of such batteries provide 15000-20000 cycles.
Besides, LTO-batteries are fireproof and resistant to cold But, LTO-batteries have several disadvantages which so far limit their use.
- It is a low specific capacity of 50-80 W / kg, whereas for traditional lithium-cobalt elements it is equal to 150-200 W / kg. this means that to obtain an equal capacity, the lithium titanate cell must be twice or three times larger.
- The nominal cell voltage is only 2.4 V versus 3.6 V in lithium-cobalt.
- Currently, lithium-titanate batteries have a high price. three times greater than that of NCA batteries.
But at the same time, lithium-titanate batteries can be used in electric buses. There is no shortage of space, and high battery life is required.
Lithium-ion-phosphate batteries
These are rechargeable lithium-ion cells that use LiFePO4 as the cathode material. The main advantage of lithium-iron-phosphate batteries over Li-ion is the increased operating temperature range. Additional benefits of the iron-phosphate battery include longer life and faster charging.
The main disadvantages of lithium-iron-phosphate batteries are lower charge density and greater sensitivity to storage at elevated temperatures.
Solid-state batteries
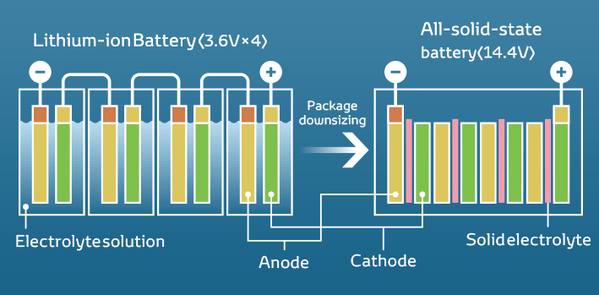
And now time to talk about solid-state batteries. So, solid-state batteries have not a liquid (gel) electrolyte, but a solid one.
The principle of their work is the same as the ionic ones. When charging, lithium ions move to the cathode, from where when the charger is turned off, they start floating freely through the electrolyte back to the anode, creating an electric current. The change in materials allows for a significant change in properties including maximum volume, charging time, size, and safety level.
The main advantages of solid-state batteries:
- Compactness: the transition from liquid to solid electrolyte provides an increase in the amount of energy for the same battery size. In the “liquid-cell” batteries, a sensible layer of liquid and a special separator separating the cathode and the anode are necessary to avoid a short circuit. For a solid-state battery, a thinner barrier is sufficient. Of course, the separator is not the only volumetric element inside the battery. for example, the cathode can be shrunk with the anode in this way. Nevertheless, approximately a solid-state battery can contain twice as much energy as a lithium-ion battery of the same thickness, or else one can say, of the same volume.
- Reliability: Solid-state electrolytes are much less prone to chemical reactions so they work longer. It also means that such batteries will not catch fire or explode due to manufacturing defects or damage.
Supercapacitors

We would especially like to highlight supercapacitors, a new energy source, which is used in electric vehicles and hybrids. Supercapacitors are significantly inferior to other traction batteries in a specific capacity. but also have undeniable advantages.
One of them is the ability to charge almost instantly in 10 to 20 seconds the charging speed is determined by the power of the charger there is an order of magnitude greater charge-discharge cycles and they can be guaranteed for 10-15 years of usage, depending on the type of supercapacitor, and its production technology.
But, they store much less energy. One charge is enough for 2 to 20 km of travel depending on the capacity of the block of supercapacitors. therefore, supercapacitors have not yet found wide application in traction installations of electric vehicles. But such vehicles have great potential for use as public transport.
For example, in the Chinese city Ningbo, there is an eco-friendly route of public transport. It is used by an electro bus that obtains relatively small motivity on the stops when the passengers board and disembark and approximately 10 seconds. There is an interesting new type of electro bus the one designed by a Chinese electric locomotive company. The company offered to equip public transport with a special saddle on the roof for a quick charge. On the stops along the route, there are supporting brackets with attaching plugs that are inserted in bus connectors one such charging provides 5 km of vehicle usage.
It works due to ultracapacitors, which are designed to be able to charge a million cycles work for 12 years, and it has working temperatures from -40 to +65 °С.
This concludes the article on electric vehicle batteries. we have learned the main electrical parameters of the traction battery. Also, we have considered the types of batteries used in electric transport and the features of their operation.



